Soap Bubbles - OPOD
Soap Bubbles: A Mesmerizing Display of Optics
Soap bubbles have long captivated our imaginations with their iridescent colors and ethereal beauty. These delicate spheres, when illuminated by light, create a mesmerizing display of optical phenomena. In this article, we will explore the fascinating world of soap bubbles and delve into the science behind their enchanting visual effects.
Reflections: A Play of Light
When light encounters a soap bubble, it undergoes multiple reflections and refractions. Each bubble reflects the surrounding scene not just once, but twice. The front side of the bubble acts as a convex mirror, similar to a car's wing mirror, producing an upright virtual image. On the other hand, the back surface of the bubble is concave, forming a real but inverted image within the bubble itself. These dual reflections contribute to the captivating visual experience of soap bubbles.
Interference: The Source of Shimmering Colors
The shimmering colors that adorn soap bubbles arise from the phenomenon of interference. The thin skin of a soap bubble consists of closely spaced layers that reflect light waves. When these waves overlap and interfere with each other, they create vibrant colors. The interference colors change with viewing angle and as the bubble's skin evaporates and thins. Rays of certain colors may be in-phase and appear brighter, while others may be out-of-phase and appear dimmer. This interplay of interference gives rise to the ever-shifting hues that dance upon the surface of soap bubbles.
Surface Tension: The Sculptor of Spheres
The spherical shape of soap bubbles is a result of surface tension, the unbalanced attraction between molecules at the surface of a liquid. The skin of a soap bubble acts like a sheet of elastic, striving to minimize its energy by assuming a shape with the least stretching possible - a sphere. This inherent property of surface tension ensures that small bubbles naturally form into perfect spheres.
Pressure and Bubble Formation
The contraction force of surface tension creates a pressure difference between the inside and outside of a soap bubble. Smaller bubbles experience greater internal pressure due to their smaller size. One might wonder how bubbles can form when a liquid boils, given that the pressure inside a bubble with zero radius would be infinite. However, when bubbles intersect, their sizes determine the shape of their shared surface. Similarly-sized bubbles have a nearly flat common surface, while a small-large bubble union causes the common surface to bulge into the larger bubble.
Foam Physics: A Complex Network
Beyond individual soap bubbles, the world of foams presents an intricate and captivating subject of study. Foams are comprised of countless soap bubbles that interact with each other, forming a complex network of surfaces. Each surface within a foam strives to minimize its energy, resulting in intricate structures. In 1887, Lord Kelvin proposed an ideal low energy structure for foams, consisting of packed 14-sided polyhedra known as tetrakaidecahedra. However, in 1994, Denis Weaire and Robert Phelan discovered a less regular structure composed of 12 and 14-sided polyhedra, which had slightly lower energy than Kelvin's foam. The quest for understanding the foam physics and identifying the structure with the least energy continues to intrigue researchers.
The Enigmatic World of Soap Bubbles
Soap bubbles provide us with a captivating window into the world of optics. These delicate spheres reflect and refract light in mesmerizing ways, showcasing the principles of reflection, interference, and surface tension. The ever-changing colors and shapes that adorn soap bubbles serve as a reminder of the complexity and beauty inherent in the natural world. So, the next time you encounter a soap bubble floating in the air, take a moment to marvel at its enchanting display and appreciate the wonders of atmospheric optics.

Soap Bubbles ~ Imaged by astrophotographer Göran Strand (web,blog).
Each bubble reflects the surrounding scene twice, once upright and another inverted. All are suffused by shimmering colours.
©G�ran�Strand, shown with permission
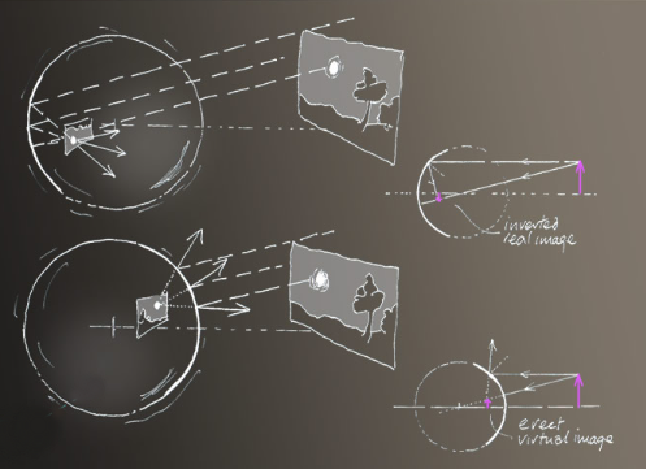
Reflections - The two reflections arise from the bubble back and front sides.
The front presents a convex surface to the scene. Think of it as similar to the convex wing mirror on a car. In it the image is erect. It is a virtual image that cannot be captured anywhere on a screen.
The back surface is concave to the scene and reflects it to form a real but inverted image within the bubble
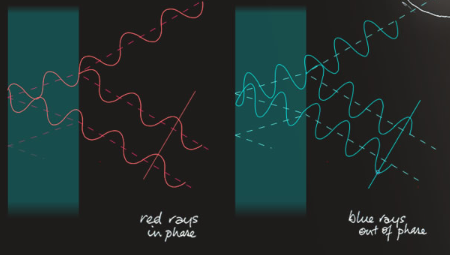
Interference - The colours come from overlap and interference between light waves reflected from the closely spaced surfaces of the bubble skin. The skin is only a few wavelengths of light or less in thickness. In a particular direction, rays of one colour might be in-phase and bright while other colour waves are less in phase and dimmer. The interference colours shift and change with viewing direction and as the bubble skin evaporates and thins.
Small bubbles are spherical because their skins "act" like sheets of elastic that try to reach a minimum energy configuration where the stretching is least - a sphere. Surface tension is the true agent, the unbalanced attraction at the surface between soap or water molecules.
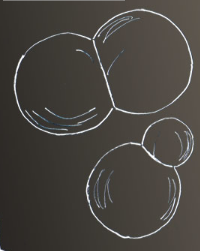
The contraction force of surface tension increases the pressure inside the bubble. The smaller the bubble the greater the excess pressure. How then can bubbles ever form when a liquid boils? The pressure inside a zero radius bubble would be infinite!
The difference in excess pressure with size show when two bubble intersect. More or less equal sized bubbles - as in G�ran's image have a near flat common surface.
A small-large bubble union has a common surface bulging into the larger of the bubbles.
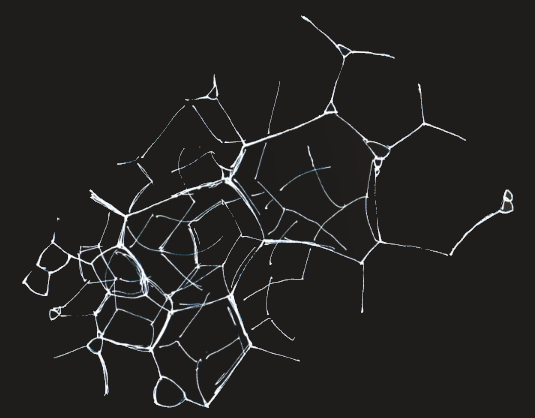
Foams get really complicated. Each surface pulls to minimise its energy.
Calculating the structure of a foam that has the lowest energy is no trivial matter. Lord Kelvin in 1887 tackled the problem and considered a foam of equal volume cells. He derived an ideal low energy structure of packed 14-sided polyhedra, tetrakaidecahedra.
There the matter stood until 1994 when Denis Weaire and Robert Phelan at Trinity College, Dublin derived a less regular structure of 12 and 14 sided polyhedra that had an energy 0.3% less than that of a Kelvin foam.
Is a Weaire‑Phelan foam the least energy? We do not know.
For a really fascinating account of foam physics try this video.
Note: this article has been automatically converted from the old site and may not appear as intended. You can find the original article here.
Reference Atmospheric Optics
If you use any of the definitions, information, or data presented on Atmospheric Optics, please copy the link or reference below to properly credit us as the reference source. Thank you!
-
<a href="https://atoptics.co.uk/blog/soap-bubbles-opod/">Soap Bubbles - OPOD</a>
-
"Soap Bubbles - OPOD". Atmospheric Optics. Accessed on November 26, 2024. https://atoptics.co.uk/blog/soap-bubbles-opod/.
-
"Soap Bubbles - OPOD". Atmospheric Optics, https://atoptics.co.uk/blog/soap-bubbles-opod/. Accessed 26 November, 2024
-
Soap Bubbles - OPOD. Atmospheric Optics. Retrieved from https://atoptics.co.uk/blog/soap-bubbles-opod/.