OPOD - Opal
OPOD - Opal: Unveiling the Beauty of Nature's Photonic Crystal
Opal, a mesmerizing gemstone, captivates with its fiery flashes of gold and emerald. However, beyond its allure lies a captivating optical phenomenon that sets it apart from other gemstones. In this article, we will delve into the intricate structure of opal and explore the science behind its vibrant display of colors.
The Amorphous Beauty of Opal
Unlike most crystals, opal is not crystalline in the conventional sense. Its structure is composed of amorphous spheres of silica (SiO2) closely packed together. These spheres mimic the ordered arrangement found in true crystals, but on a much larger scale.
The opal sphere lattices lack the strict uniformity seen in crystalline structures. The spheres vary in size, which disrupts the close packing observed in a box of uniform marbles. When marbles of different sizes are introduced, the regular arrangement is disrupted, resulting in small groups or no arrangement at all.
Precious Opal: A Kaleidoscope of Colors
Precious opal, highly sought after for its vibrant flashes of color, possesses spheres with diameters ranging from 150 to 300 nanometers. To put this into perspective, the wavelength of red light measures approximately 600 nanometers. Within precious opal, there are macro-sized domains where the spheres are relatively uniform in size and form a fairly orderly tetragonal, cubic, or hexagonal lattice. These domains are responsible for the captivating color display exhibited by precious opal.
The colors in opal arise from the interference between light waves scattered by each individual sphere. As the regularly spaced spheres scatter light waves of certain wavelengths, their crests overlap constructively, strengthening the scattered light. However, wavelengths that do not experience constructive interference do not contribute to the observed colors. Consequently, patches of intense color emerge, with the specific hues depending on the size and spacing of the silica spheres within the small domains.
Unveiling the Science Behind Opal's Colors
From a mathematical standpoint, opal domains can be better understood as a regular three-dimensional array with a periodically varying refractive index. This unique structure classifies opal as a photonic crystal. In essence, a photonic crystal transmits certain wavelengths of light while blocking others, creating band gaps where transmission is impeded. The wavelengths that are blocked are scattered back out of the crystal, resulting in the vibrant colors we perceive.
Interestingly, photonic crystals can also be found elsewhere in nature. Peacock feathers, for example, derive their stunning colors from two-dimensional photonic crystals. Various organisms such as butterflies, beetles, and fish exhibit similar optical phenomena. The potential applications of photonic crystals extend beyond their natural occurrence and into the realm of opto-electronics and other scientific fields.
Exploring the Boundless Potential of Photonic Crystals
Researchers are investing considerable effort into fabricating opal and other photonic crystal structures. Moreover, they have even developed "inverse-opal" structures, where the spaces between the spheres are filled, allowing for visualization of the intricate lattice. By dissolving away the silica spheres, scientists can uncover the underlying architecture and gain further insight into the behavior of light within these extraordinary materials.
In conclusion, opal stands as a testament to nature's ingenuity in creating captivating optical phenomena. Its amorphous structure, composed of closely packed silica spheres, forms a photonic crystal that scatters and selectively transmits light, resulting in the breathtaking display of colors. As researchers continue to explore the boundless potential of photonic crystals, we can anticipate exciting advancements in opto-electronics and other fields, inspired by the awe-inspiring beauty found within opal and other natural wonders.

Opal amongst quartz crystals flashes fire, gold and emerald. Imaged from her mineral collection by Barbara Grudzinska (flickr) of Warsaw, Poland.
Image ©Barbara Grudzinska, shown with permission.
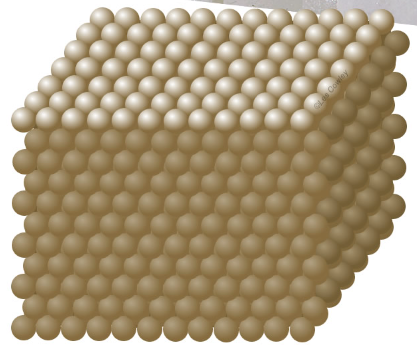

Idealised precious opal structure.
The spheres are amorphous silica 150-300nm diameter. Each sphere scatters light. The individual outgoing waves interfere. Some colours interfere constructively, other destructively. Collectively the spheres form a photonic crystal
Opal is not crystalline in the usual sense. It is made of amorphous spheres of silica (SiO2) packed closely together. The sphere packing only mimics the extremely ordered arrangement of the much smaller single atoms and ions in the lattice of a true crystal.
Opal sphere lattices are far less ordered. The spheres are of varied size and thus they do not pack together well. Take a box of uniform sized marbles and rattle it well too and fro � the marbles will arrange into a close packed array. Add a few marbles of different size and this no longer happens, there will only be small groups, if any, regularly arranged. The out of size marbles disrupt the close packing.
Precious or gem opal, sought for its flashes of colour, has spheres 150 � 300 nm diameter. In comparison red light has a wavelength of ~600nm. Precious opal has macro sized domains where the spheres are fairly uniform in size and, locally at least, pack into a fairly orderly tetragonal, cubic or hexagonal lattice. These are the domains that can flash colour.
Mechanistically, the colour arises from interference between light waves scattered by each sphere. Since the spheres are regularly spaced, scattered light waves of certain wavelengths will overlap with their waves crests matching. The scattered light will be strengthened. Other wavelengths are less fortunate, their outgoing wave crests do not overlap constructively and their colours are not seen. The result is patches of intense colour . The colour depends on the silica ball size and thus spacing in the small domain.
Mathematically, the colours and directionality are better predicted by regarding an opal domain as a regular 3D array of periodically varying refractive index - which it is. This is a photonic crystal. It transpires that the crystal will transmit some wavelengths but there are also band gaps where transmission is blocked. The blocked wavelengths are scattered back out of the crystal � these are the intense colours that we see.
Photonic crystals arise elsewhere in Nature, for example a peacock�s colours are from 2D crystals. Some butterflies, beetles and fish have them. Photonic crystals have enormous potential application in opto-electronics and elsewhere. Great effort is going into fabricating opal and other structures and even making 'inverse-opal' which can be visualised by filling all the spaces between the balls at right and then dissolving away the spheres!
Note: this article has been automatically converted from the old site and may not appear as intended. You can find the original article here.
Reference Atmospheric Optics
If you use any of the definitions, information, or data presented on Atmospheric Optics, please copy the link or reference below to properly credit us as the reference source. Thank you!
-
<a href="https://atoptics.co.uk/blog/opod-opal/">OPOD - Opal</a>
-
"OPOD - Opal". Atmospheric Optics. Accessed on November 27, 2024. https://atoptics.co.uk/blog/opod-opal/.
-
"OPOD - Opal". Atmospheric Optics, https://atoptics.co.uk/blog/opod-opal/. Accessed 27 November, 2024
-
OPOD - Opal. Atmospheric Optics. Retrieved from https://atoptics.co.uk/blog/opod-opal/.