Chemistry of Banded Airglow - OPOD
The Chemistry Behind Banded Airglow
Airglow, a phenomenon that illuminates the night sky, is a captivating display of natural light. It is primarily emitted by electronically excited oxygen atoms, which acquire their energy from the sun's extreme ultraviolet radiation through a series of absorption and collisional events. One particular form of airglow known as banded airglow has intrigued scientists and sky enthusiasts alike. In this article, we will delve into the intricate chemistry behind banded airglow and explore its mesmerizing characteristics.
The green light emitted by banded airglow originates from a thin layer situated approximately 90 to 100 kilometers above the Earth's surface. This layer corresponds to the emission of radiation from the transition of oxygen atoms from the 1S state to the 1D state. Interestingly, this radiation is considered "forbidden" according to the quantum rules governing everyday radiation. However, the quantum world defies absolutes, and the green light manages to escape within a mere second, comparable to the age of the Universe in atomic terms. Alternatively, these excited oxygen atoms can lose their energy through collisions with other atoms and molecules present in the atmosphere.
The narrowness of the emitting green layer in banded airglow arises from a delicate balance between radiative and collisional decay. At lower altitudes where pressures are higher, collisions prevail, inhibiting the formation of the narrow green layer. Conversely, at higher altitudes, where there are fewer 1S atoms, collisions become less frequent, allowing the radiative decay process to dominate and giving rise to the distinct banding pattern observed in banded airglow.
In addition to the green light emitted by oxygen atoms transitioning from the 1S state to the 1D state, banded airglow also exhibits red light emissions. These red emissions result from transitions between excited oxygen atoms in the 1D state and the 3P state. Unlike the green emissions, the red emissions occur at much higher altitudes, ranging from 150 to 300 kilometers above the Earth's surface. At these rarefied heights, the excited oxygen atoms can survive for significantly longer periods, emitting light for an impressive radiative lifetime of approximately 110 seconds.
The distinctive bands observed in banded airglow owe their existence to gravity waves propagating upwards from disturbances in the troposphere. These waves modulate the density of the airglow layer, effectively switching the airglow on or off. As the waves pass through the layer, they create regions of higher and lower density, leading to the formation of the characteristic banded pattern.
To understand the chemistry behind banded airglow, it is essential to delve into the electronic configuration of oxygen atoms. Oxygen atoms possess eight electrons distributed among various energy states represented by spectroscopic shorthand. These energy states are associated with different "orbitals," which can be visualized as shapes where the electron probability concentrates. Oxygen atoms have two inner "s" orbitals and three mutually perpendicular "p" orbitals. The arrangement of electrons within these orbitals determines the electronic configuration and influences the atom's properties.
The lowest energy state, known as 3P or "triplet p," involves one orbital occupied by two electrons of opposite spin, while the remaining two electrons with the same spin reside in separate orbitals. The next energetic state, referred to as 1D or "singlet dee," consists of paired electrons of opposite spin occupying two orbitals. Finally, the highest energy state, denoted as 1S, involves electrons of opposite spin singly occupying two orbitals.
These subtle variations in electronic configuration significantly impact the chemistry of the upper atmosphere and give rise to the vibrant colors observed in airglow and aurorae. The transitions between these different electronic states result in the emission of green and red light, contributing to the captivating beauty of banded airglow.
In conclusion, banded airglow is a captivating atmospheric phenomenon that showcases the intricate interplay between excited oxygen atoms and their electronic configurations. The emission of green and red light at specific altitudes, coupled with the influence of gravity waves, gives rise to the distinct banding pattern observed in the night sky. By unraveling the chemistry behind banded airglow, scientists gain valuable insights into the behavior of atoms in the upper atmosphere and further our understanding of the mesmerizing optical displays that grace our skies.

Airglow Chemistry Intense banded airglow pictured by Yuri Beletsky at Carnegie Las Campanas Observatory in the Atacama Desert, Chile. "We just witnessed pretty amazing airglow here in Chile! It was the night of March, 17-18, and the airglow's intensity was so high that we could easily see its structure by eye - in B&W of course". � Image ©Yuri Beletsky, shown with permission
The airglow is emitted by electronically excited oxygen atoms. Its energy comes from the sun�s extreme ultraviolet radiation transferred to the oxygen by a number of absorption and collisional events.
A thin layer 90 to 100 km high emits the green light. The radiation (O 1S to 1D ) is not allowed by the quantum rules for everyday radiation and is said to be �forbidden�. But the quantum world has few absolutes and the radiation oozes out within about a second � a time comparable to the age of the Universe in atomic terms. The atoms can lose their energy in another way � via collisions with other atoms and molecules. The narrowness of the emitting green layer is the result of competition between radiative and collisional decay. At lower altitudes pressures are higher and collisions win. Higher up there are too few 1S atoms.
Other excited oxygen atoms emit the red light (1D to 3P radiation) at altitudes of 150 � 300km. Only at these rarefied heights can these atoms survive long enough to emit light for their radiative lifetimes are even longer � an immense 110 seconds.
The Bands result from waves (gravity waves) propagating upwards from disturbances in the troposphere. The waves modulate the density at the airglow height and switch the airglow on or off.
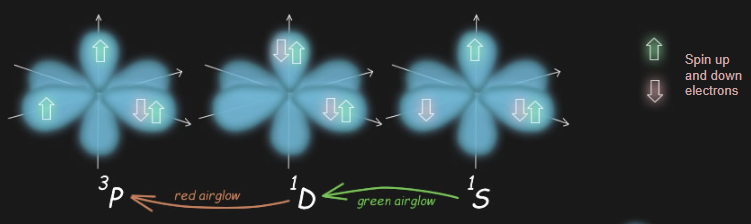
The symbols 1S, 1D and 3P are spectroscopic shorthand for an atom's precise electronic configuration.
Oxygen atoms have eight electrons. These have different states represented by �orbitals�. Orbitals can be thought of as shapes where most of the electron probability concentrates. They have different forms. �s� orbitals are spherical, �p� orbitals have two lobes and come in triplets mutually perpendicular. There are others more exotic.
Two of the oxygen�s electrons occupy an inner lowest energy 's' orbital. Two more occupy an outer 's' orbital. Things then get more interesting. The next higher energy orbitals for occupancy are three mutually perpendicular 'p' orbitals. Electrons have spin (call it up or down) and any one orbital can only have a single electron (up or down) or a pair (up and down). There are therefore three ways that electrons can arrange in oxygen�s p orbitals.
The lowest energy state (3P - 'triplet 'p'') has one orbital occupied by two electrons of opposite spin. The remaining two electrons of the same spin are each in a separate orbital.
The next most energetic state (1D - 'singlet dee') has paired electrons of opposite spin in two orbitals.
The highest energy (1S) has electrons of opposite spin singly occupying two orbitals.
These subtle electronic variations alter the atom's properties and have a major influence on upper atmosphere chemistry. Transitions between the states give us the greens and reds of airglow and the beauty of aurorae.
None of the structures and rules are arbitrary. They come wholly naturally from solutions of the Schrodinger equation (or its equivalents) for an atom with bound energy states.

Atomic 'p' orbitals. There are three at right angles and, in the absence of electric or magnetic fields they have the same energy
Note: this article has been automatically converted from the old site and may not appear as intended. You can find the original article here.
Reference Atmospheric Optics
If you use any of the definitions, information, or data presented on Atmospheric Optics, please copy the link or reference below to properly credit us as the reference source. Thank you!
-
<a href="https://atoptics.co.uk/blog/chemistry-of-banded-airglow-opod/">Chemistry of Banded Airglow - OPOD</a>
-
"Chemistry of Banded Airglow - OPOD". Atmospheric Optics. Accessed on November 26, 2024. https://atoptics.co.uk/blog/chemistry-of-banded-airglow-opod/.
-
"Chemistry of Banded Airglow - OPOD". Atmospheric Optics, https://atoptics.co.uk/blog/chemistry-of-banded-airglow-opod/. Accessed 26 November, 2024
-
Chemistry of Banded Airglow - OPOD. Atmospheric Optics. Retrieved from https://atoptics.co.uk/blog/chemistry-of-banded-airglow-opod/.