Blue Airglow
Blue Airglow: Exploring the Mysteries of the Night Sky
Have you ever gazed up at the night sky and wondered about the captivating phenomenon known as blue airglow? While it may not be as well-known as its green and red counterparts, blue airglow adds a touch of enchantment to the nocturnal canvas. In this article, we will delve into the depths of this ethereal phenomenon, shedding light on its origins, characteristics, and the science behind its mesmerizing glow.
The Elusive Blue Airglow
Blue airglow is a relatively rare occurrence that is often overshadowed by its more prominent counterparts. Unlike the vibrant hues of green and red airglow, blue airglow is much fainter and more elusive. It is primarily observed at an altitude of approximately 95 kilometers, where excited molecular oxygen plays a vital role in its formation.
Unraveling the Enigma
The process of blue airglow formation is rather intricate and fascinating. It occurs through indirect excitation of molecular oxygen at an altitude of around 95 kilometers. This excitation can be triggered by various pathways, such as the dissociation of N2 and NO during daylight or the recombination of NO+ during twilight. These reactions ultimately generate excited O2 molecules, which emit blue multi-wavelength banded radiation known as Herzberg bands.
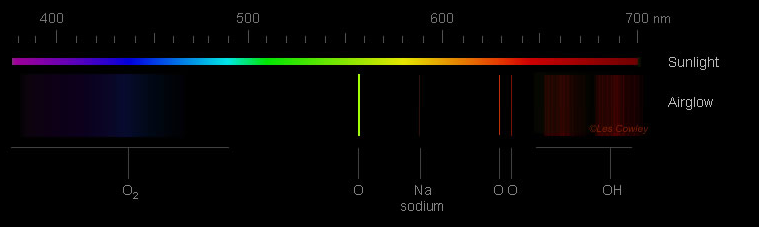
The Dim Glow of Airglow
Airglow itself is a phenomenon that occurs throughout the Earth's atmosphere. It is characterized by a faint glow caused by the excitation of atoms and molecules. While our eyes are unable to perceive its colors due to its faintness, cameras are capable of capturing its subtle beauty. The most common color of airglow is green, resulting from the light emitted by atomic oxygen at an altitude of 90-100 kilometers. Additionally, red airglow can also be observed at higher altitudes, around 150-300 kilometers, caused by another forbidden transition of oxygen atoms. At a lower altitude of approximately 86 kilometers, excited OH radicals emit a red glow in a narrow layer.
The Luminous Dance of the Upper Atmosphere
The upper atmosphere is a stage for various luminous performances, each with its own unique characteristics. Aurorae, the most well-known atmospheric phenomenon, create a stunning display of colors primarily visible at high latitudes. These are caused by the interaction of solar wind electrons and protons with atoms and molecules in the upper atmosphere. In contrast, airglow occurs due to the excitation of atoms and molecules by solar far ultraviolet radiation. While aurorae are more prominent and widely observed, airglow has the potential to be visible from all latitudes if not for the interference of light pollution.
Capturing the Elusive Glow
Photographing blue airglow presents a significant challenge due to its faintness and rarity. In fact, few have been fortunate enough to witness and document this captivating phenomenon. Professional astrophotographer Doug Zubenel managed to capture a stunning image of blue airglow during his expedition to photograph Comet Tuttle passing by the Pinwheel Galaxy M33. His photograph showcased a patch of red airglow at the upper left corner, accompanied by a mesmerizing blue glow adjacent to it.
A Sky Filled with Mysteries
As we gaze up at the night sky, it becomes apparent that there is still much to learn and explore. The captivating dance of colors and phenomena like blue airglow remind us of the vastness and complexity of our universe. Each discovery brings us closer to unraveling the mysteries that lie beyond our reach.
In conclusion, blue airglow is a rare and enchanting phenomenon that adds a touch of magic to the nocturnal sky. Its formation involves the indirect excitation of molecular oxygen at an altitude of approximately 95 kilometers. While airglow itself is a widespread occurrence, blue airglow is much fainter and harder to observe. Nevertheless, the few fortunate enough to witness its mesmerizing glow are treated to a sight that ignites curiosity and wonder. As we continue to explore the wonders of the night sky, let us remember that there is always more to discover and marvel at in the vast expanse above us.

Blue Airglow
Imaged by Doug Zubenel 70 miles south of Kansas City in the St. Philippine Duchesne Memorial Park, Linn County on December 30, '07. ©Doug Zubenel, shown with permission.
"This is the only instance of blue airglow I have ever imaged .more of Doug’s airglow photographs 1,2,3. I was actually making exposures of the zodiacal light following a very successful expedition to photograph Comet Tuttle passing by the Pinwheel Galaxy M33. None of the airglow was visible to the naked eye. At upper left is a patch of red airglow, and adjacent to the left side of the blue area is some pink.
As far as I know, all the trails in the photo are jets - the short ones are just farther away. Being near Kansas City we have tons of air traffic.
The crosses are memorials to the more than 600 native Americans that perished during a forced move from the Great Lakes region to Oklahoma at the end of the 19th century.
Guided, 84 second exposure at ~ 7:33 pm CST ( 01:33 UT on the December 31) with a 16mm NIKKOR fisheye @ f/4 at ISO 800. The RAW file was converted to a TIFF and auto colour correction applied in PhotoShop. I have worked with this camera and these softwares enough to know that blue is present."
The upper atmosphere glows. Two processes light it. The brightest, visible mostly at high latitudes, is the glow of atoms and molecules excited by collisions with solar wind electrons and protons injected downwards from Earth�s nightside magnetosphere � Aurorae. The dimmer glow, visible in principle if our light pollution would allow from all latitudes, is also from excited atoms and molecules, this time the excitation is ultimately from solar far ultra violet radiation � Airglow.
Airglow is so faint that our eyes do not see colour. Cameras do and the most common is green from the light of atomic oxygen 90-100 km high. Red airglow is also seen. That at 150-300 km high is from another forbidden transition of oxygen atoms. Lower down, in a narrow layer at ~86 km, excited OH radicals also glow red.
Blue airglow is much much fainter. Excited molecular oxygen at ~95 km high can produce it. The excitation is indirect. Possible routes are via daylight dissociation of N2 and NO or twilight recombination of NO+ whose reaction products generate excited O2. The oxygen then decays by emitting blue multi-wavelength banded radiation (Herzberg bands) if it is not first collisionally de-excited.
Note: this article has been automatically converted from the old site and may not appear as intended. You can find the original article here.
Reference Atmospheric Optics
If you use any of the definitions, information, or data presented on Atmospheric Optics, please copy the link or reference below to properly credit us as the reference source. Thank you!
-
<a href="https://atoptics.co.uk/blog/blue-airglow/">Blue Airglow</a>
-
"Blue Airglow". Atmospheric Optics. Accessed on November 25, 2024. https://atoptics.co.uk/blog/blue-airglow/.
-
"Blue Airglow". Atmospheric Optics, https://atoptics.co.uk/blog/blue-airglow/. Accessed 25 November, 2024
-
Blue Airglow. Atmospheric Optics. Retrieved from https://atoptics.co.uk/blog/blue-airglow/.