Auroral Oxygen
Auroral Oxygen: Unveiling the Enigmatic Green Glow
Have you ever witnessed the mesmerizing dance of the Northern Lights, also known as the Aurora Borealis? These ethereal displays of light in the night sky have captivated humans for centuries. One of the most striking features of the Aurora Borealis is the predominant green color that illuminates the heavens. This vibrant hue is a result of the 557.7 nm wavelength light emitted by oxygen atoms transitioning from an excited state to a lower energy state.
The process behind this phenomenon, known as "auroral oxygen," is both fascinating and complex. Unlike the green airglow, which is excited by the sun's extreme ultraviolet light, auroral oxygen is energized through collisions with high-energy particles that are accelerated downwards into the Earth's atmosphere from the night side magnetotail. These particles originate from the sun, primarily during powerful solar maximum X-Class flares.
However, the presence of solar particles alone would not be sufficient to generate the captivating auroral displays we witness. Solar protons, electrons, and heavier ions reaching the Earth's vicinity are typically not energetic enough. It is only under specific magnetic and energetic conditions that these solar particles enter the sunward side of Earth's magnetosphere and get swept back by magnetic forces into the plasma mantle of the million-kilometer-long night-side magnetotail. There, they remain trapped until magnetic rearrangements accelerate some of them violently downwards.
The collision between these accelerated particles and oxygen atoms in the Earth's atmosphere results in the excitation of the oxygen atoms. As they return to a lower energy state, they emit photons of light at a wavelength of 557.7 nm, creating the stunning green glow that characterizes auroral oxygen.
Jan Curtis, an avid photographer, captured an extraordinary image of the Alaska Aurora on the evening of November 27, 2000. The photograph beautifully showcases the vibrant green color of auroral oxygen against the dark night sky. Curtis took this captivating image just two miles west of the University of Fairbanks, Alaska, using a Nikon FE camera and Nikkor f1.4 lens at 35mm. The exposure was set to 12 seconds at f/2.0, and the film used was Kodak Royal Gold 400 print film.
Understanding the science behind auroral oxygen not only deepens our appreciation for the beauty of the Northern Lights but also sheds light on the intricate interplay between the Earth, its magnetosphere, and the sun. By studying these phenomena, scientists can gain valuable insights into the behavior of particles in space and their effects on our planet.
To summarize the key points about auroral oxygen:
- The green color in the Northern Lights is primarily due to the emission of light at a wavelength of 557.7 nm by excited oxygen atoms transitioning to a lower energy state.
- Unlike green airglow, which is excited by the sun's extreme ultraviolet light, auroral oxygen is excited by high-energy particles accelerated downwards into the Earth's atmosphere from the night side magnetotail.
- Solar particles, such as protons, electrons, and heavier ions, are not typically energetic enough to generate aurorae on their own.
- Specific magnetic and energetic conditions allow solar particles to enter the sunward side of Earth's magnetosphere and get swept back into the plasma mantle of the night-side magnetotail.
- The collision between accelerated particles and oxygen atoms in the Earth's atmosphere leads to the excitation of oxygen atoms and the subsequent emission of green light.
- Jan Curtis's photograph of the Alaska Aurora beautifully captures the vivid green glow of auroral oxygen against the night sky.
- Understanding auroral oxygen contributes to our knowledge of Earth's magnetosphere and its interaction with solar particles.
- Scientists study these phenomena to gain insights into the behavior of particles in space and their impact on our planet.
The allure of the Northern Lights continues to inspire awe and wonder in those fortunate enough to witness their splendor. As we delve deeper into the science behind auroral oxygen, we uncover the intricate mechanisms that produce these celestial displays, enriching our understanding of the Earth's connection to the cosmos.

Alaska Aurora imaged by Jan Curtis (Aurora site)) Fairbanks, Alaska on the evening of November 27, 2000. ©Jan Curtis, shown with permission.
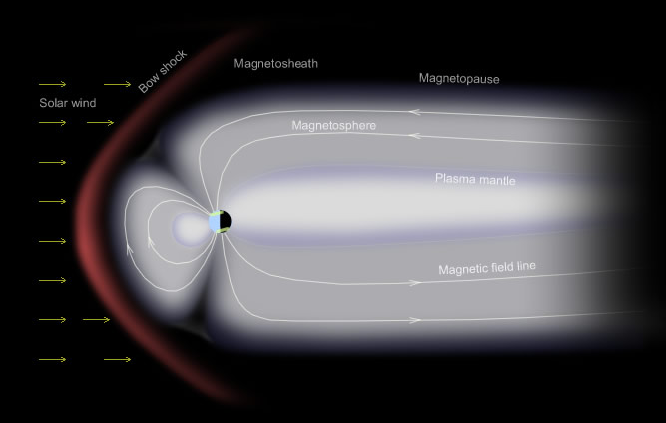
The predominant green colour is the 557.7 nm wavelength light of oxygen atoms decaying from an excited state to another of lower energy (O1S -> 1D). The same electronic transition is responsible for green airglow but the way the oxygen atoms are excited is very different. The oxygen of airglow is excited by the sun's extreme ultraviolet light. Auroral oxygen is excited by collisions with high energy particles accelerated downwards into the atmosphere from the earth's night side magnetotail. The particles derive initially from the sun (in this display from powerful solar maximum X-Class flares) but, on their own, we would see few aurorae because solar protons, electrons and heavier ions reaching the earth's vicinity are ordinarily not nearly energetic enough.
Under the right magnetic and energetic conditions the solar particles enter the sunward side of earth's magnetosphere and are swept back by magnetic forces into the plasma mantle of the million km long night-side magnetotail. There they are are trapped until magnetic rearrangements accelerate some of them violently downwards.
Jan Curtis took the image two miles west of the University of Fairbanks, Alaska at 9:25PM local time with a Nikon FE camera and Nikkor f1.4 lens at 35mm, exposure 12 s @ f/2.0 onto Kodak Royal Gold 400 print film.
Note: this article has been automatically converted from the old site and may not appear as intended. You can find the original article here.
Reference Atmospheric Optics
If you use any of the definitions, information, or data presented on Atmospheric Optics, please copy the link or reference below to properly credit us as the reference source. Thank you!
-
<a href="https://atoptics.co.uk/blog/auroral-oxygen/">Auroral Oxygen</a>
-
"Auroral Oxygen". Atmospheric Optics. Accessed on November 26, 2024. https://atoptics.co.uk/blog/auroral-oxygen/.
-
"Auroral Oxygen". Atmospheric Optics, https://atoptics.co.uk/blog/auroral-oxygen/. Accessed 26 November, 2024
-
Auroral Oxygen. Atmospheric Optics. Retrieved from https://atoptics.co.uk/blog/auroral-oxygen/.