Aurorae Colours - OPOD
Aurorae Colours - Exploring the Phenomenon
Aurorae, also known as the Northern and Southern Lights, are mesmerizing natural light displays that occur in the polar regions. These breathtaking spectacles are caused by interactions between charged particles from the Sun and Earth's magnetic field. While we often admire the vibrant colors of the aurorae, have you ever wondered about the science behind these captivating hues? In this article, we will delve into the intricate details of aurorae colors and unravel the secrets behind their mesmerizing beauty.
The Colorful Culprits
The key players responsible for the vivid colors of aurorae are oxygen atoms, nitrogen atoms, and molecular nitrogen (N2). When energetic particles from Earth's magnetotail collide with these atmospheric constituents during solar storms, they become electronically excited, leading to the emission of light.
- Atomic oxygen, found at altitudes of 100-150 km, produces an intense green color. This vibrant shade is a result of the electronic excitation caused by collisions with energetic particles.
- At even greater altitudes, between 150 and 500 km, collisions become less frequent. In this region, another excited state of oxygen can survive long enough to radiate red light, adding a touch of crimson to the auroral display.
- Molecular nitrogen also contributes to the color palette, although to a lesser extent. Its excited ion emits a purple-blue hue at very high altitudes. In particularly intense auroral events, a red-purple fringe can be observed beneath the glowing green curtains of atomic oxygen.
The Science Behind the Colors
The variation in altitude and electronic transitions within the atmospheric constituents gives rise to the distinct colors observed in aurorae. Let's explore the scientific principles behind this phenomenon:
- The red light emitted by oxygen (singlet D to ground state triplet P transition) is observed at higher altitudes compared to the green light. This is due to the longer lifetime of the singlet D state transition, allowing it to occur at greater heights.
- Both the red and green transitions are spin forbidden, resulting in exceptionally long lifetimes. As a result, these colors are visible only at very low pressures where collisions cannot quench the excitation first.
- Interestingly, ionized molecular nitrogen emission can be observed above both the red and green layers, adding an additional layer of complexity to the auroral display.
Capturing the Beauty
Witnessing an aurora is a truly awe-inspiring experience, but capturing its ethereal beauty can be equally rewarding. Photographers from around the world strive to capture the vivid colors and intricate patterns displayed in the night sky. To immortalize these breathtaking moments, photographers often employ advanced techniques such as High Dynamic Range (HDR) imaging.
Parag Mahajani, an avid photographer from Pune, India, beautifully captured the aurorae in Iceland using a Canon DSLR 550D and a 50 mm lens. His images were further enhanced using Photomatix Pro 5.0, allowing for stunning HDR compositions that showcase the full range of colors and details present in the auroral display.
Conclusion
The colors of aurorae are a testament to the complex interplay between energetic particles, Earth's magnetic field, and atmospheric constituents. From the intense green of atomic oxygen to the delicate hues of ionized molecular nitrogen, each color adds depth and vibrancy to these celestial phenomena. The science behind aurorae colors continues to captivate researchers and photographers alike, inspiring us to marvel at the wonders of our planet and the universe beyond. So, the next time you witness the dancing lights of an aurora, take a moment to appreciate the intricate science behind its captivating colors.

Auroral Colours, Iceland
Images by Parag Mahajani of Pune, India. Canon DSLR 550D, 50 mm lens, 15-60s. HDR images using Photomatix Pro 5.0.
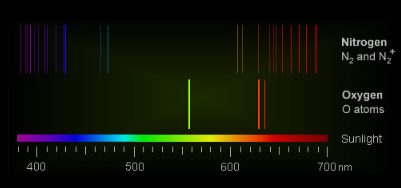
The culprits responsible for auroral colours are oxygen atoms, nitrogen atoms and molecular nitrogen (N2). They are electronically excited by collisions with energetic particles spilling from Earth’s magnetotail overfilled during solar storms.
Atomic oxygen at 100-150 km produces intense green. At greater altitudes of 150 to 500 km collisions are so infrequent that another oxygen excited state can survive enough to radiate red light.
Molecular nitrogen is a smaller contributor. Its excited ion gives purple-blue at very high altitudes and sometimes, in violent displays, a red-purple fringe below the green curtains of glowing atomic oxygen.

The red light of oxygen (singlet D to ground state triplet P) is evidently at greater altitude than its green and shorter lifetime singlet S to singlet D state transition.
Both transitions are spin forbidden hence they have very long lifetimes and we only see this light at very low pressures where collisions cannot quench the excitation first.
A hint of ionised molecular nitrogen emission tops both.

Note: this article has been automatically converted from the old site and may not appear as intended. You can find the original article here.
Reference Atmospheric Optics
If you use any of the definitions, information, or data presented on Atmospheric Optics, please copy the link or reference below to properly credit us as the reference source. Thank you!
-
<a href="https://atoptics.co.uk/blog/aurorae-colours-opod/">Aurorae Colours - OPOD</a>
-
"Aurorae Colours - OPOD". Atmospheric Optics. Accessed on November 26, 2024. https://atoptics.co.uk/blog/aurorae-colours-opod/.
-
"Aurorae Colours - OPOD". Atmospheric Optics, https://atoptics.co.uk/blog/aurorae-colours-opod/. Accessed 26 November, 2024
-
Aurorae Colours - OPOD. Atmospheric Optics. Retrieved from https://atoptics.co.uk/blog/aurorae-colours-opod/.