Labradorite ~ Structural Colour
Labradorite: The Stunning World of Structural Colour
Labradorite, a mesmerizing feldspar mineral, holds a secret within its depths - an intense display of colors that seem to emanate from its core. When held under a bright light and gently rotated, flashes of peacock blue, vibrant greens, golden hues, and rustic tones come alive. This captivating phenomenon is known as "labradorescence," a unique example of structural coloration.
Unlike traditional pigments that absorb light, labradorescence derives its hues from wave interactions within nanostructures. These nanostructures can range from a simple soap film to the intricate biological structures found in butterfly wings, peacock displays, and the shimmering scales of fish. Labradorite's iridescent colors are produced by multi-layer interference within its crystal lattice.
The formation of labradorite begins when it solidifies from a melt. As it cools, two silicate compositions, albite (NaAlSi3O8) and anorthite (CaAl2Si2O8), which were miscible at high temperatures, become incompatible and separate into two phases. These phases arrange themselves into stacked alternate layers or lamellae, which are responsible for the mesmerizing iridescence.
To understand how structural coloration works, we can look at the example of soap bubbles and oil films. When light strikes a single layer, part of it is reflected from the front surface, while another part enters the film, reflects off the rear surface, and leaves in the same direction as the front reflected wave. The combination of these outgoing waves creates either constructive interference (resulting in light) or destructive interference (resulting in darkness), depending on the alignment of their wave crests.
In the case of labradorite, the presence of multiple layers enhances this interference phenomenon. Light leaving the structure undergoes several reflections between the layers, each with its own interference possibilities. The alternate layers of albite-rich and anorthite-rich compositions in labradorite have a small difference in refractive index, leading to weak reflections at the layer boundaries. Consequently, a large number of layers are required to produce an intense reflection, resulting in a narrow bandwidth or wavelength range of the reflected light and the perception of nearly pure colors.
The complexity of predicting and understanding the behavior of multilayer films is evident due to the intricate network of possible wave paths. In 1917, Lord Rayleigh, a prominent figure in optics and mathematical physics, developed a theory to explain these phenomena. Today, modern methods rely on numerical calculations and form an essential part of the growing field of optics and communications.
Labradorite's structural coloration is a testament to the wonders of scientific understanding. Rather than dulling or diminishing nature's beauty, scientific knowledge enhances our appreciation of its marvels. As poet John Keats famously wrote, "A thing of beauty is a joy forever," and labradorite's structural coloration exemplifies this sentiment by showcasing the harmonious fusion of nature's artistry and scientific principles.
In conclusion, labradorite's labradorescence offers us a glimpse into the captivating world of structural coloration. Its intricate layers and wave interactions create a symphony of vivid hues that dance before our eyes. As we hold this remarkable mineral under the light, we are reminded of the beauty that can arise from the interplay between science and nature. Labradorite truly embodies the enchantment and allure of structural color, leaving us in awe of its radiant splendor.

"Labradorescence" � Intense colours shine from deep inside the mineral labradorite. Imaged by Kevin Boyle. ©Kevin Boyle, shown with permission.
Hold labradorite under a bright light and slowly rotate it. From deep within come flashes of peacock blue, greens, golds and rusts.
Labradorite is a feldspar, a sodium aluminium silicate with 50 to 70% of the sodium sites in the crystal lattice replaced by calcium. After it solidified from a melt, labradorite cooled and silicate compositions, albite NaAlSi3O8 and anorthite CaAl2Si2O8, that were miscible at high temperatures became incompatible and separated into two phases. The phases are arranged in stacked alternate layers or lamellae and it is these that produce the iridescent colours by multi-layer interference.
Labradorescence is an example of �structural colour� where hues derive not from pigment absorption but from wave interactions in nanostructures. These range in complexity from a single soap film to biological structures of astounding subtlety. The latter give the vivid hues of some butterfly wings, a peacock�s display and the silvery scales of fishes.
Interference across a single layer produces the colours of soap bubbles and oil films. Light striking the layer is part reflected from the front surface. Another part enters the film, reflects off the rear surface and leaves in the same direction as the front reflected wave.
The two outgoing waves combine. If their wave crests (shown red for positive and blue for negative at left) coincide then there is light. When they are out of phase there is destructive interference and there is darkness. The phase condition is wavelength dependent with the result that we see interference colours.
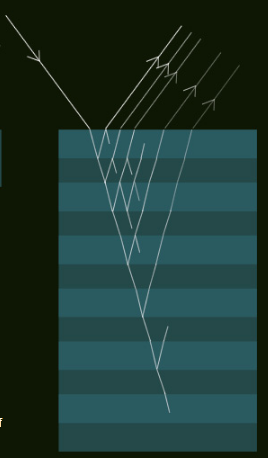
All this is enhanced when there are multiple layers. Light leaving the structure is the result of several reflections between layers, each with interference possibilities.
In Labradorite the alternate albite rich and anorthite rich layers have only a small difference in refractive index. Reflections at layer boundaries are weak and a large number of layers are needed to produce an intense reflection. This is an advantage because the bandwidth or wavelength range of the reflected light narrows and we see an almost pure colour. The bandwidth roughly halves for a doubling of the number of layers. Tens perhaps hundreds of layers contribute to the intense colour flashes from deep within the stone.
Computing and predicting how multilayer films behave is complicated. Not surprising considering the many branching network of possible wave paths to be considered. Lord Rayleigh, the giant of optics and mathematical physics, developed a theory in 1917. Modern methods are numerical and are part of an important and growing area of optics and communications.
We can unweave the rainbow and for that matter a labradorite crystal also. But contrary to poet Keats' oft quoted assertions, �charms .do not. fly at the mere touch of cold philosophy�. Scientific understanding enhances Nature�s wonder and beauty rather than clips or dulls it.
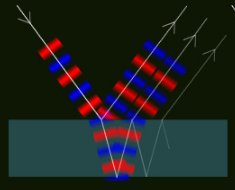
Single film interference:
Waves reflected from the two sides of the film combine and interfere. In-phase waves give light. The phase condition is wavelength and angle dependent. Hence we see coloured reflections in different directions.
Multiple film interference:
The interference principles are the same. There are more opportunities for interference. With the right layer thicknesses and properties, reflections of particular colours are intense and pure.
Note: this article has been automatically converted from the old site and may not appear as intended. You can find the original article here.
Reference Atmospheric Optics
If you use any of the definitions, information, or data presented on Atmospheric Optics, please copy the link or reference below to properly credit us as the reference source. Thank you!
-
<a href="https://atoptics.co.uk/blog/labradorite-structural-colour/">Labradorite ~ Structural Colour</a>
-
"Labradorite ~ Structural Colour". Atmospheric Optics. Accessed on April 23, 2024. https://atoptics.co.uk/blog/labradorite-structural-colour/.
-
"Labradorite ~ Structural Colour". Atmospheric Optics, https://atoptics.co.uk/blog/labradorite-structural-colour/. Accessed 23 April, 2024
-
Labradorite ~ Structural Colour. Atmospheric Optics. Retrieved from https://atoptics.co.uk/blog/labradorite-structural-colour/.