Glowing Gases - Aurorae
Glowing Gases - Aurorae: A Spectacular Natural Phenomenon
Aurorae, also known as the Northern and Southern Lights, are one of the most captivating natural phenomena that grace our planet's skies. These mesmerizing displays of colorful lights are caused by the interaction of energetic particles from the magnetotail with the Earth's upper atmosphere, specifically the thermosphere. While the existing content provides some insights into the nature of aurorae, let's delve deeper into the details to truly appreciate the beauty and complexity of these glowing gases.
The Colors of Aurorae
The colors observed in aurorae are a result of the excited states of various atoms and molecules in the upper atmosphere. The predominant color observed at low altitudes is green, which is emitted by electronically excited oxygen atoms. As the altitude increases, the color transitions to red. This change occurs due to excited nitrogen molecules and nitrogen molecular ions, which produce pink and red hues.
Energetic Particle Interactions
Energetic particles from the magnetotail, such as electrons and protons, spiral downwards along magnetic lines of force, penetrating deep into the Earth's upper atmosphere. These particles collide with atoms and molecules in the upper atmosphere, leading to processes such as ionization, dissociation, and excitation. These interactions result in the formation of glowing aurorae.
Oxygen's Role in Auroral Light
Oxygen atoms play a crucial role in the production of auroral light. Above 100 km in altitude, the atmosphere primarily consists of oxygen atoms and nitrogen molecules. Solar extreme ultraviolet light dissociates molecular oxygen into atoms. The green light observed in aurorae comes from highly energetic oxygen atoms that decay from a higher energy level to a lower, but still excited energy level. The radiative lifetime of these excited atoms is approximately one second, which is relatively long compared to ordinary electronic transitions. However, collisions with other atoms and molecules often cause the excited oxygen atoms to lose their energy, making green radiation possible only in the near vacuum of the upper atmosphere.
The Elusive Red Aurorae
In addition to green, oxygen atoms are also responsible for the occurrence of red aurorae. The red light emitted by oxygen atoms is even more elusive than the green light. It originates from less excited oxygen atoms decaying to oxygen's lowest electronic level. The radiative lifetime of these atoms is an immense 110 seconds, but they can only radiate above 150 km in altitude. At lower altitudes, their energy is typically lost in collisions before they have a chance to emit red light.
Altitude Ranges of Auroral Colors
Different altitudes correspond to the distinct colors observed in aurorae. Green oxygen aurorae are typically observed between 100 km and 150 km in altitude. As the altitude increases, red oxygen aurorae become visible, spanning from 150 km upwards to 250 km. In rare cases, red oxygen aurorae can even extend beyond 600 km.
Nitrogen's Contribution to Aurorae
Molecular nitrogen (N2) is another major constituent of the thermosphere. However, due to its exceptional stability, there are relatively few nitrogen atoms below 400 km in altitude that contribute to the formation of aurorae. The faint green emission from these nitrogen atoms is often masked by the more prominent green light emitted by oxygen. However, in intense displays, a deep red-violet border can be observed beneath the usual green curtains. This border arises from excited molecular nitrogen, while nitrogen molecular ions produce purple-blue aurorae at very high altitudes.
The Challenges of Observing Aurorae
Observing aurorae is an awe-inspiring experience, but it is not without its challenges. To witness these beautiful displays, we must peer through tens to hundreds of kilometers of glowing gas. At sea level, the atmosphere is considered a vacuum compared to the upper atmosphere where aurorae occur. For example, at an altitude of 100 km, the atmospheric pressure is approximately one millionth of that at sea level. Oxygen atoms at this altitude experience an average distance of about one meter between collisions, undergoing approximately 500 collisions per second. This high collision rate quickly removes any excitation. At 200 km, where red oxygen aurorae glow, the atmosphere becomes even more rarefied. Oxygen atoms travel an average distance of 4 to 5 kilometers between collisions and are hit, on average, only once every 7 seconds. This increased time between collisions allows excited atoms to radiate their energy, resulting in the soft and elusive glow characteristic of aurorae.
Beyond Green and Red: Other Auroral Phenomena
While green and red are the predominant colors observed in aurorae, there are other fascinating phenomena associated with these celestial displays. Proton aurorae, for instance, are rarer than their electron-induced counterparts. The particle energies involved in aurorae range from 1 to 100 KeV, which is far greater than the energy of the original solar wind particles. These high energies contribute to the vibrant and dynamic nature of auroral displays.
The Quantum Secrets Behind Auroral Colors
The emission of specific colors in aurorae is governed by quantum selection rules. For example, the transition responsible for the green light observed in oxygen aurorae is known as the O 1S to 1D transition. This transition is considered "forbidden" according to quantum selection rules for electric and magnetic dipole transitions. Consequently, the transition probability is very low, resulting in slow decay and the production of radiation within a very narrow band.
In conclusion, aurorae are a breathtaking natural phenomenon that captivates viewers with their vibrant colors and ethereal glow. The interaction of energetic particles with the Earth's upper atmosphere gives rise to these awe-inspiring displays. From the predominantly green and red hues emitted by excited oxygen atoms to the faint contributions of nitrogen, aurorae continue to fascinate scientists and spectators alike. Observing these atmospheric wonders reminds us of the dynamic and interconnected nature of our planet and the universe beyond.
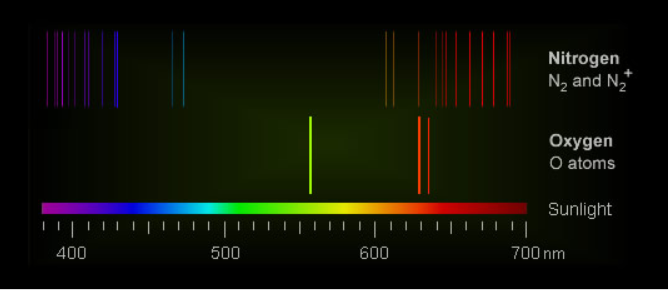
Auroral light is mostly from electronically excited oxygen atoms. Green radiation prevails at low altitudes and red at higher.
Excited nitrogen molecules and nitrogen molecular ions produce pink and red at low altitudes.
Energetic particles from the magnetotail spiral downwards along magnetic lines of force to penetrate deep into Earth’s tenuous upper atmosphere, the thermosphere. The most energetic. reach down to ~80 km (50 mile). They collide.. with the upper atmosphere’s atoms and molecules producing ionisation, dissociation and excitation. The clouds of excited atoms eventually radiate their excess energy to form the glowing shifting aurorae.
Most auroral light is from excited oxygen atoms. Above 100 km the atmosphere is mainly oxygen atoms and nitrogen molecules, the molecular oxygen is dissociated into atoms by solar extreme ultraviolet light.
The auroral green light is a single extremely narrow wavelength (557.7 nm) from very energetic oxygen atoms decaying to a lower, but still excited energy level.... The radiative lifetime of the excited atoms is about a second and the decay is slow, an eternity by ordinary electronic transition standards. In that time many of the excited atoms lose their energy instead by collisions with other atoms and molecules. The green radiation is only possible in the near vacuum of the upper atmosphere where collisions are less frequent. Also, there are few oxygen atoms below 100 km to produce it.
Oxygen atoms are also responsible for red aurorae. If oxygen’s green radiation is emitted grudgingly, its red light is even more so. The radiation is from less excited atoms decaying to oxygen’s lowest electronic level^. Their radiative lifetime is an immense 110 seconds and the atoms only have a chance to radiate above 150 km. At lower altitudes their energy is nearly always first lost in collisions.
Green oxygen aurorae are at 100 km up to about 150 km. Red oxygen aurorae are 150 km upwards to 250 km and more rarely to 600 km plus.
The other major thermosphere constituent, molecular nitrogen N2, is exceptionally stable and there are not many nitrogen atoms below 400 km to make aurorae. The few nitrogen atoms emit a faint green masked by that of oxygen. In very intense displays there is a deep red violet border beneath the usual green curtains. This is emission from excited molecular nitrogen. Nitrogen molecular ions produce purple blue aurorae at very high altitudes.
We only see aurorae because we are looking through tens to hundreds of kilometres of glowing gas. By sea level standards that 'gas' is a vacuum. At 100 km, the altitude of green aurorae, the atmosphere's pressure is a millionth of that at sea level and the mean distance an oxygen atom travels between collisions is about a metre^^. Even so, it undergoes about 500 collisions each second and any excitation is quickly removed. At 200 km, where the red oxygen aurora glows, the vacuum has hardened. The oxygen atom will travel on average 4 to 5 kilometre between collisions and will be hit on average only once every 7 seconds. Excited atoms then have ample time time to radiate their energy and their collective light over a layer perhaps tens of kilometres thick gives us the soft and elusive auroral glow. Lights in a vacuum.. ..almost!
. Most excitation is by collisions with electrons. proton aurorae are rarer.
.. The particle energies range from 1 to 100 KeV - far greater than that of the original solar wind particles.
...
The transition is O 1S to 1D. The singlet S to singlet D state transition is not allowed by the quantum selection rules for electric and magnetic dipole transitions. The consequences are a very low transition probability, slow decay and very narrow band radiation. The transition is said to be 'forbidden'.
^ O 1D to ground state 3P transitions, also forbidden.
^^ A gas is an ensemble of particles with a range of velocities defined by the temperature and the particle masses. The greater the temperature the greater the spread of velocities and the greater their absolute values. The distances quoted are 'mean free paths' defined by gas kinetic theory. They are approximate and depend on temperature, pressure, and the gas moleculat weight.
Note: this article has been automatically converted from the old site and may not appear as intended. You can find the original article here.
Reference Atmospheric Optics
If you use any of the definitions, information, or data presented on Atmospheric Optics, please copy the link or reference below to properly credit us as the reference source. Thank you!
-
<a href="https://atoptics.co.uk/blog/glowing-gases-aurorae/">Glowing Gases - Aurorae</a>
-
"Glowing Gases - Aurorae". Atmospheric Optics. Accessed on December 22, 2024. https://atoptics.co.uk/blog/glowing-gases-aurorae/.
-
"Glowing Gases - Aurorae". Atmospheric Optics, https://atoptics.co.uk/blog/glowing-gases-aurorae/. Accessed 22 December, 2024
-
Glowing Gases - Aurorae. Atmospheric Optics. Retrieved from https://atoptics.co.uk/blog/glowing-gases-aurorae/.