Airglow Formation
Airglow Formation: A Fascinating Display of Atmospheric Optics
Airglow is a captivating phenomenon that illuminates the night sky with a subtle glow. It occurs when atoms and molecules in the Earth's atmosphere become excited and emit light. In this article, we will delve deeper into the intricacies of airglow formation, exploring its various components, its relationship with aurorae, and the factors that influence its intensity and variability.
Components of Airglow
Airglow is primarily composed of electronically and/or vibration-rotationally excited atoms and molecules that reside at an altitude of 80 kilometers (50 miles) or higher. The dominant source of airglow is oxygen atoms, which emit green light at a wavelength of 558 nanometers. This emission is visible from Earth's orbit and is responsible for the most prominent feature of airglow. Additionally, there is a weaker red light emitted by oxygen atoms at higher altitudes, further contributing to the overall glow. Other contributors to airglow include sodium atoms, hydroxyl radicals (OH), and molecular oxygen.
Distinction from Aurorae
While both airglow and aurorae are manifestations of excited atoms in the atmosphere, there are notable differences between the two phenomena. Aurorae occur at similar altitudes as airglow but are primarily caused by collisions with energetic particles rather than chemical excitation by short-wavelength solar radiation. In the case of airglow, the excitation is predominantly driven by the sun's extreme ultraviolet (EUV) radiation, leading to the chemical excitation of oxygen atoms as the primary component.
The Role of Solar Radiation
The sun's EUV radiation plays a crucial role in airglow formation. When this high-energy radiation interacts with oxygen and nitrogen atoms and molecules in the thermosphere, it excites them. These energized particles then collide with other atmospheric components, including hydroxyl radicals (OH), resulting in a cascade of chemical reactions. Eventually, this process leads to light emission through a phenomenon known as chemiluminescence. The decay of excited atoms and molecules also contributes to the production of light.
The Green Glow of Atomic Oxygen
The most visually striking component of airglow is the green light emitted by oxygen atoms in a layer located between 90 and 100 kilometers (56-62 miles) above the Earth's surface. This emission is exceptionally bright and easily observable from space. However, the emission does not occur at lower altitudes due to the intense collisional quenching, reduced intensity of extreme UV sunlight, and a lower concentration of oxygen atoms.
The Enigmatic Red Airglow
In addition to the green glow, airglow also exhibits a mesmerizing red light emitted by atomic oxygen. This red radiation originates from a lower energy excited state with an extraordinarily long radiative half-life of 110 seconds. Consequently, this red airglow is only present at higher altitudes, specifically between 150 and 300 kilometers (93-186 miles), where collisions are infrequent enough to allow the excited atoms sufficient time to radiate away their energy. This red emission is closely related to the emission from OH radicals.
Sodium and Other Components
Another significant contributor to airglow is the familiar yellow light emitted by sodium atoms in a layer situated at an altitude of 92 kilometers (57 miles). This yellow glow, which was previously attributed to the upward transport of sea salt, has been found to originate from gas phase bicarbonate (NaHCO3) reacting with oxygen atoms. Additionally, weak blue emissions from excited molecular oxygen at around 95 kilometers (59 miles) and red and infrared emissions from vibrationally and rotationally excited OH radicals at approximately 86-87 kilometers (53-54 miles) further enhance the complexity of airglow.
Variability and Non-Uniformities
Airglow is not a static phenomenon but rather exhibits variability in both intensity and spatial distribution. It can display bands and patches that shift and vary over short periods of time, often just minutes. These variations are influenced by gravity waves propagating from the lower atmosphere, which modulate the atmospheric density, temperature, and composition at airglow altitudes. Consequently, these waves impact the intensity of airglow emissions.
Diurnal Changes and Solar Activity
The brightness of airglow is highest on the day side of Earth, where the initial excitation occurs. On the night side, airglow is significantly fainter, approximately one thousandth as bright as its daytime counterpart. Furthermore, airglow exhibits variations over longer timescales due to the 11-year cycle of solar activity. As solar activity waxes and wanes, it affects the intensity and characteristics of airglow.
A Window into Atmospheric Composition
The study of airglow provides valuable insights into the composition and dynamics of Earth's atmosphere. By analyzing the emissions and their characteristics, scientists can gain a deeper understanding of the interactions between different atmospheric components and the impact of solar radiation on our planet. Additionally, the non-uniformities and variability observed in airglow serve as a reminder of the intricate and ever-changing nature of our atmosphere.
In conclusion, airglow formation is a captivating display of atmospheric optics that involves the excitation and emission of atoms and molecules in Earth's upper atmosphere. With its vibrant green and enigmatic red emissions, airglow adds a touch of mystique to our night sky. By unraveling its complex components and studying its behavior, scientists continue to unlock the secrets of our atmosphere and expand our knowledge of the world above us.
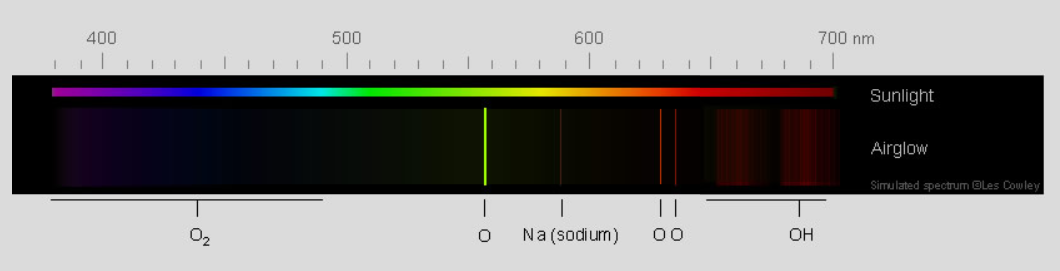
Airglow Spectrum - Green light from excited oxygen atoms dominates the glow. The atoms are 90-100 km (56-62 mile) high in the thermosphere. The weaker red light is from oxygen atoms further up. Sodium atoms, hydroxyl radicals (OH) and molecular oxygen add to the light.
The airglow is the light of electronically and/or vibration-rotationally excited atoms and molecules 80 km or higher.
Airglow vs Aurorae
Aurorae are at similar heights and are also the light of excited atoms. There is a difference, however, auroral excitation is by collisions with energetic particles whereas daytime short wavelength solar radiation produces the airglow via chemical excitation of which electronically excited oxygen atoms are the main component.
Production by sun's EUV radiation
The sun’s extreme ultraviolet light excites oxygen and nitrogen atoms and molecules in the thermosphere.. The energetic products collide and interact with other atmospheric components, including hydroxyl radicals (OH), to eventually produce light emission by chemiluminescence.. and and decay of excited atoms and molecules.
Atomic oxygen green radiation
The brightest emission is green 558nm light from oxygen atoms in a layer 90-100 km high. The emission layer is clearly visible from earth orbit.
Radiation vs collisional de-excitation
The excited atoms take about a second to decay to another lower energy excited state.... By atomic emission standards this is extremely slow and in that time many excited atoms lose their energy instead by collisions, mainly with nitrogen molecules. The emission does not occur at lower altitude because the collisional quenching is so severe, the extreme UV sunlight is less intense and there are fewer oxygen atoms
Atomic oxygen
red light The red radiation of atomic oxygen is from a lower energy excited state whose radiative half-life is an immensely long, 110 seconds^. This red airglow is only found at 150 - 300 km where collisions are so infrequent that the excited atoms have time to radiate away their energy. See also the red emission from OH radicals below..
Sodium Another airglow component is the familiar yellow light from sodium atoms^^ in a layer at 92 km.
O2 There are weak blue emissions from excited molecular oxygen at ~95 km.
OH Vibrationally and rotationally excited OH radicals emit red (image) and infra-red in a narrow layer (6-10 km FWHM) centered at ~ 86-87 km^^^.
Non uniformities Airglow is not always uniform. It can have bands and patches which shift and vary over minutes. Gravity waves propagating from the lower atmosphere modulate the atmospheric density, temperature and composition at airglow altitudes and thus the airglow intensity.
Diurnal changes
Solar 11 year cycle The airglow is brightest on Earth's day side where the original excitation occurs. The night airglow is (fortunately!) only one thousandth as bright and varies through the night. On a much longer timescale the airglow varies with the 11 year cycle of solar activity.
. Above 100 km the atmosphere is mainly oxygen atoms and nitrogen molecules, molecular oxygen is dissociated into atoms by the solar extreme ultraviolet light.
.. Chemiluminescence is light emitted during a chemical reaction or later from the excited products of a reaction.
... The transition is O 1S to 1D. The singlet S to singlet D state transition is not allowed by quantum selection rules for dipole transitions. The transition probability is consequently low and the decay slow. The radiation is said to be 'forbidden'.
^ The two red photons are from O 1D to ground state 3P transitions, also forbidden.
^^ In contrast, the yellow sodium Na 2P to 2S transitions are selection rule permitted and occur very rapidly indeed. LIDAR studies show the sodium to be of meteoric origin rather than upwards transport of sea salt as previously thought. The sodium is present as gas phase bicarbonate (NaHCO3) and is activated by reaction with oxygen atoms.
^^^ The O2 and OH emissions are (Meinel) bands of many closely spaced wavelengths because the transitions involve changes in vibrational energy together with smaller changes in rotational eneergy. The excited OH source is the Bates-Nicolet reaction between ozone and hydrogen atoms. The OH airglow is limited at higher altitudes by the rapid fall off in ozone concentration with height and at lower levels by the onset of rapid quenching of the excited products by collisions more frequent at the higher atmospheric pressures. The balance between the two limiting processes creates the narrow OH airglow layer.
Note: this article has been automatically converted from the old site and may not appear as intended. You can find the original article here.
Reference Atmospheric Optics
If you use any of the definitions, information, or data presented on Atmospheric Optics, please copy the link or reference below to properly credit us as the reference source. Thank you!
-
<a href="https://atoptics.co.uk/blog/airglow-formation/">Airglow Formation</a>
-
"Airglow Formation". Atmospheric Optics. Accessed on December 24, 2024. https://atoptics.co.uk/blog/airglow-formation/.
-
"Airglow Formation". Atmospheric Optics, https://atoptics.co.uk/blog/airglow-formation/. Accessed 24 December, 2024
-
Airglow Formation. Atmospheric Optics. Retrieved from https://atoptics.co.uk/blog/airglow-formation/.