Freezing Bubbles, Slovakia - OPOD
Freezing Bubbles: A Fascinating Natural Phenomenon in Slovakia
Have you ever witnessed the captivating sight of freezing bubbles? Daniela Rapava, a keen observer at the Rimavská Sobota Observatory in Slovakia, managed to capture this enchanting natural phenomenon. In a frigid environment with temperatures around minus 5 Celsius, Daniela's bubble mixture, consisting of 1-2 volumes of sugar, 2 volumes of water, and 3 volumes of detergent, froze before her eyes.
When water undergoes a phase change from liquid to solid, it requires tiny nuclei or seeds on which ice crystals can grow. Without these nuclei, the water remains in a higher Gibbs energy state as a supercooled liquid. However, at extremely low temperatures, typically below -40 degrees Celsius, homogeneous nucleation occurs even in pure water.
As the freezing process unfolds, crystal growth is constrained by the thin film surrounding the bubble. The ice crystals take on plate-like habits with a remarkable six-fold hexagonal symmetry. Each crystal grows along three bi-directional growth axes, while growth along the fourth axis is limited. This geometric pattern echoes the underlying atomic structure of ice.
In addition to ice formation, another intriguing aspect is the possible crystallization of sugar within the bubbles. By observing the growth of sugar crystals on a microscope slide using crossed polarized light, one can witness a mesmerizing spectacle.
The vibrant colors seen in the frozen bubbles may be attributed to thin film interference and/or birefringence. These optical phenomena arise due to the interaction of light with the thin film of ice and the underlying structural properties of the bubbles.
The hexagonal structure of ice can be visualized using a chemist's ball and stick model. The large red balls represent oxygen atoms, while the smaller white sticks represent hydrogen atoms. Each hydrogen atom forms a tight covalent bond with one oxygen atom and a weaker bond with the other. This arrangement gives ice an open, lower density structure, allowing it to float and supporting life on Earth.
Witnessing the freezing of bubbles is not only a visually stunning experience but also a reminder of the intricate and captivating aspects of the natural world. It serves as a testament to the beauty that can be found in even the simplest of phenomena. So, the next time you encounter freezing temperatures, consider exploring this delightful activity and immerse yourself in the enchanting world of freezing bubbles.

Freezing Bubbles
Water caught in the act freezing by Daniela Rapava at Rimavská Sobota Observatory, Slovakia. The air was at about minus 5 Celsius.
Daniela’s bubble mixture was quite concentrated, 1-2 volumes sugar, 2 volumes water and 3 of detergent.
The phase change from water to ice or crystallization needs tiny nuclei, seeds, on which crystals can grow. Otherwise the water stays in a higher Gibbs energy state as a supercooled liquid. Eventually, homogeneous nucleation will occur at temperatures of -40 C or even well below in pure water.
Crystal growth is restricted by the thin film into plate-like habits with strong six-fold hexagonal symmetry. Each crystal grows along three (bi-directional) growth axes. Growth along the fourth (c) axis is restricted. The geometry echoes the underlying structure of ice at the atomic level.
At some stage the sugar could also be crystallizing out. Observe the fascinating growth of the latter on a microscope slide with crossed polarized light.
The colours could be thin film interference and/or birefringence.
Bubble blowing is fun!
All images ©Daniela Rapava
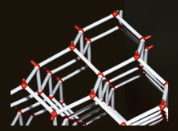
A chemist's ball and stick model of ice showing its hexagonal structure.
The red balls represent large oxygen atoms. Between them along the white sticks but not equidistant are hydrogen atoms.
Each hydrogen is tightly (covalently) bonded to one oxygen and much less so to the other. That the latter weak bonding occurs at all gives ice an open, lower density, structure that allows ice to float and us to exist.


Note: this article has been automatically converted from the old site and may not appear as intended. You can find the original article here.
Reference Atmospheric Optics
If you use any of the definitions, information, or data presented on Atmospheric Optics, please copy the link or reference below to properly credit us as the reference source. Thank you!
-
<a href="https://atoptics.co.uk/blog/freezing-bubbles-slovakia-opod/">Freezing Bubbles, Slovakia - OPOD</a>
-
"Freezing Bubbles, Slovakia - OPOD". Atmospheric Optics. Accessed on December 22, 2024. https://atoptics.co.uk/blog/freezing-bubbles-slovakia-opod/.
-
"Freezing Bubbles, Slovakia - OPOD". Atmospheric Optics, https://atoptics.co.uk/blog/freezing-bubbles-slovakia-opod/. Accessed 22 December, 2024
-
Freezing Bubbles, Slovakia - OPOD. Atmospheric Optics. Retrieved from https://atoptics.co.uk/blog/freezing-bubbles-slovakia-opod/.